


Choose ELIQUIS® for simple dosing in NVAF
ELIQUIS (apixaban): Simple dosing for stroke prevention in NVAF, with or without food
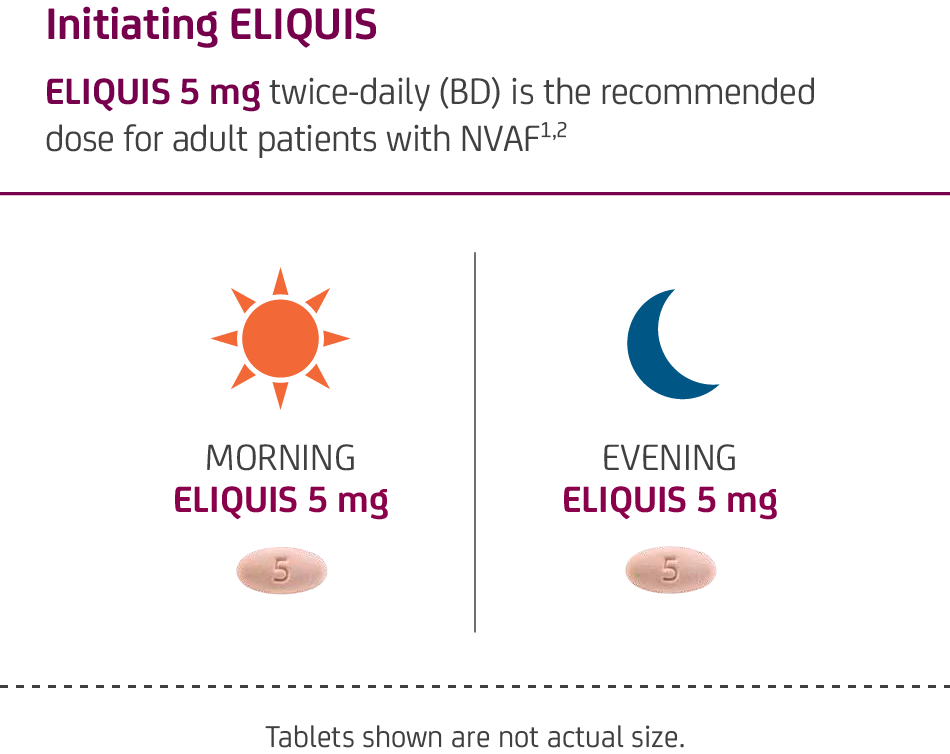
ELIQUIS can be taken
with or without food1
Missed dose: if a dose is missed,
the patient should take ELIQUIS
immediately and then continue
with twice-daily intake as before.1
ElIQUIS is not recommended for patients with creatinine
clearance <15 ml/min, or in patients undergoing dialysis.1
Please refer to the ELIQUIS Summary of Product Characteristics
for full information.1
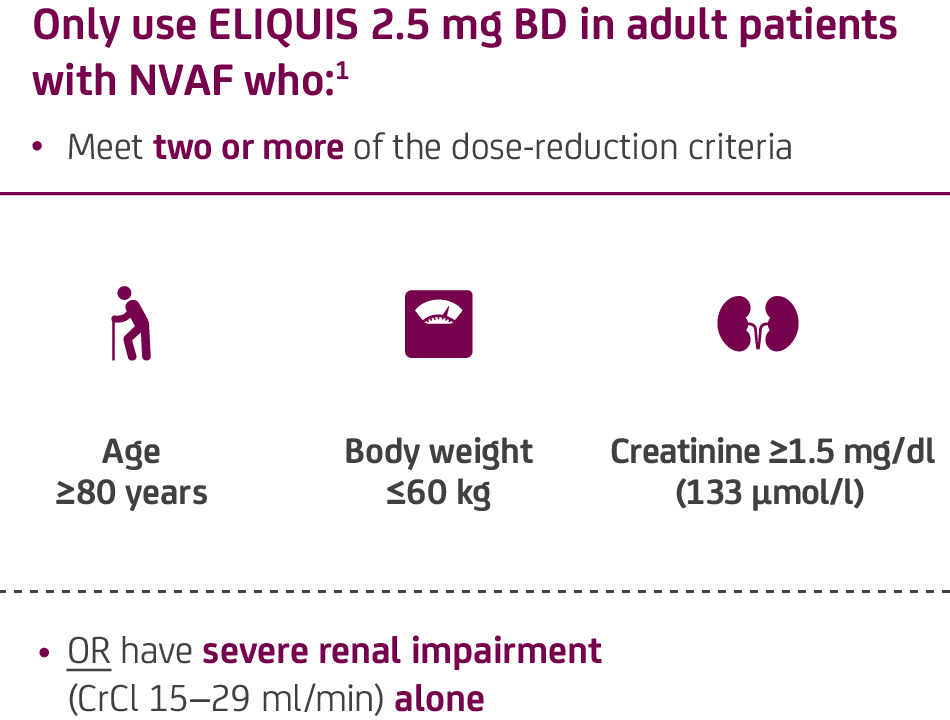
In the ARISTOTLE clinical trial, the majority of patients in the ELIQUIS group were on ELIQUIS 5 mg BD (95.3%, n=8,692 / 9,120)2*
Liver function testing should be performed prior to initiating ELIQUIS1
ELIQUIS (apixaban) is indicated for prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation (NVAF), with one or more risk factors, such as prior stroke or transient ischaemic attack (TIA), age ≥75 years, hypertension, diabetes mellitus, symptomatic heart failure (NYHA Class ≥II); treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults; and, prevention of VTE in adult patients who have undergone elective hip or knee replacement surgery.1
Footnotes:
- * In the ARISTOTLE trial, a total of 18,201 patients with NVAF and one risk factor for stroke and mean CHADS2 score of 2.1 were randomised to ELIQUIS 5mg BD (or 2.5 mg BD in selected patients [4.7%]) or warfarin adjusted to target INR range 2.0–3.0.2 Stroke or systemic embolism was the primary efficacy endpoint of the ARISTOTLE trial (haemorrhagic stroke and ischaemic or undetermined stroke were components of stroke) and major bleeding defined per International Society on Thrombosis and Haemostasis (ISTH) criteria were the primary endpoint.2 Efficacy analyses based on the intention-to-treat population. Safety analyses based on patients who had at least one dose of study drug.2
- BD = Twice Daily CHADS2 = Congestive heart failure, Hypertension, Age ≥75, Diabetes, Stroke (doubled) CrCl = Creatinine Clearance INR = International Normalised Ratio ISTH = International Society on Thrombosis and Haemostasis NVAF = Non-Valvular Atrial Fibrillation
References:
- ELIQUIS® (apixaban) Summary of Product Characteristics.
- Granger CB et al. N Engl J Med 2011; 365: 981–992.
Reporting of suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance at www.hpra.ie
Adverse reactions should also be reported to Bristol-Myers Squibb Medical Information on 1 800 749 749 or medical.information@bms.com
