


AMPLIFY-EXT Clinical Trial
Choose ELIQUIS® for prevention of recurrent DVT and PE
In AMPLIFY-EXT, in patients receiving extended treatment for prevention of recurrent DVT and PE, ELIQUIS 2.5 mg BD demonstrated superior efficacy and no significant difference in the rates of both major and major/CRNM bleeding vs placebo1,2*
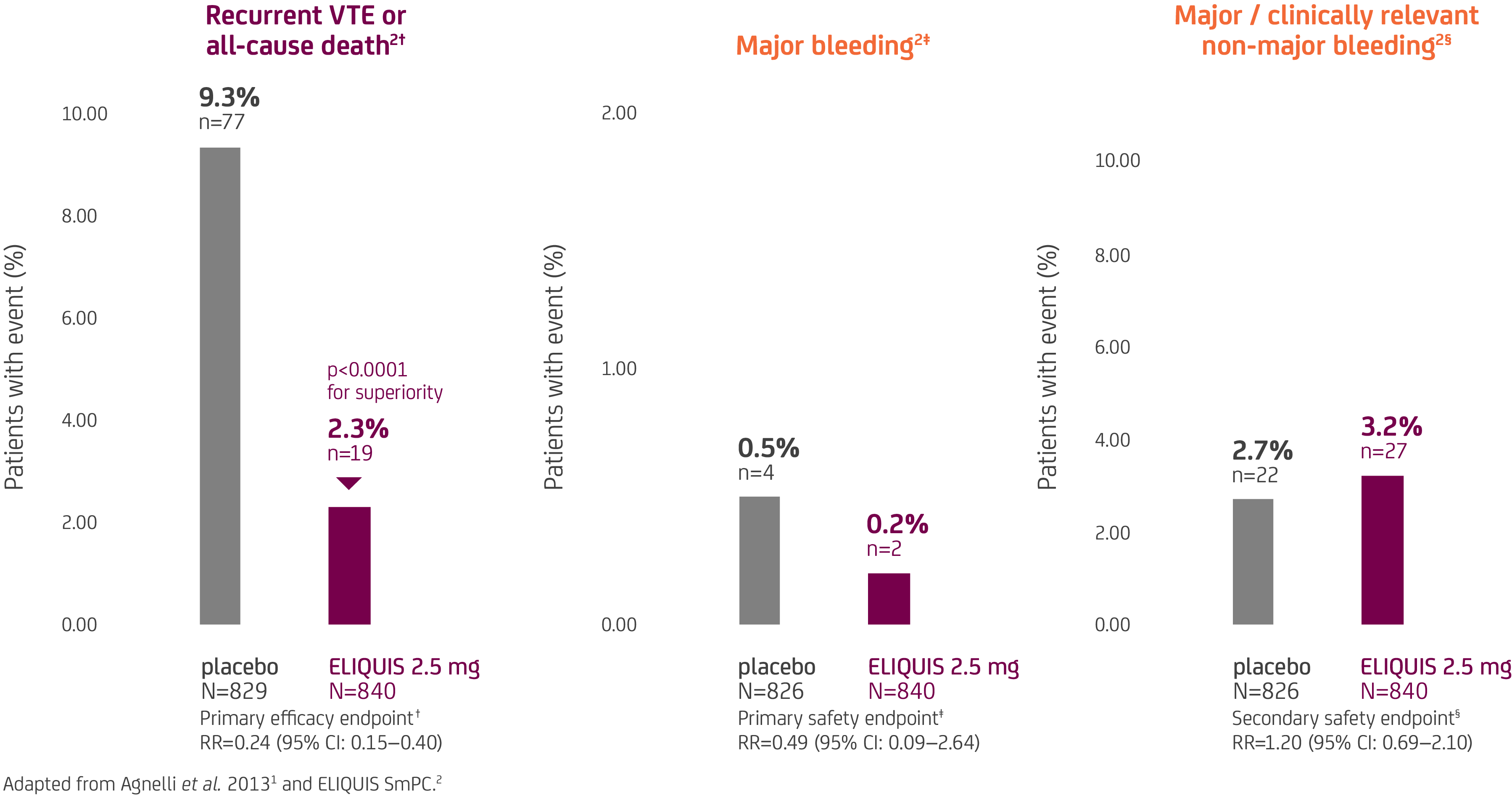
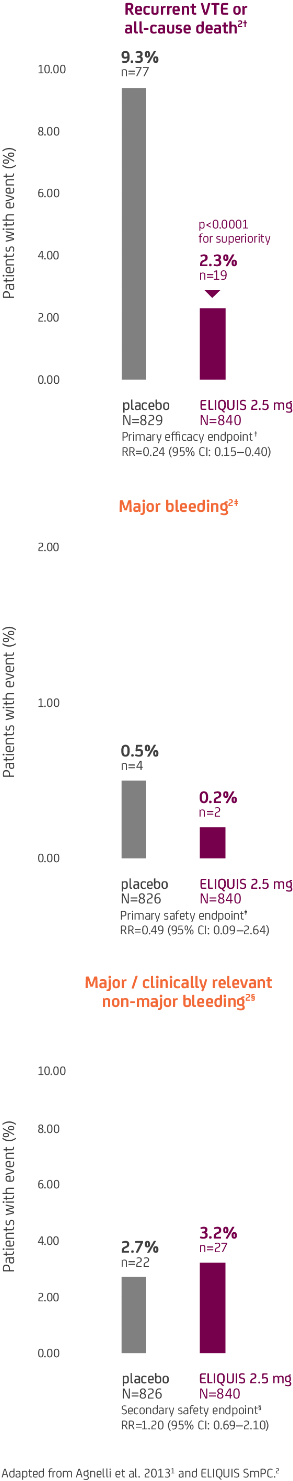
ELIQUIS is only licensed for prevention of recurrent VTE at a dose of 2.5 mg BD and initiated after 6 months of treatment with ELIQUIS 5 mg BD or another anticoagulant.2
Visit this page for more information on ELIQUIS dosing for DVT / PE.
ELIQUIS (apixaban) is indicated for prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation (NVAF), with one or more risk factors, such as prior stroke or transient ischaemic attack (TIA), age ≥75 years, hypertension, diabetes mellitus, symptomatic heart failure (NYHA Class ≥II); treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults; and, prevention of VTE in adult patients who have undergone elective hip or knee replacement surgery.2
Footnotes:
- *The AMPLIFY-EXT clinical trial was a 12 month, randomised, double-blind trial in 2,482 patients with VTE who had been treated for 6–12 months with standard anticoagulation therapy or had completed treatment with ELIQUIS or enoxaparin / warfarin as participants in the AMPLIFY trial and for whom there was clinical uncertainty about the benefit of continuing treatment.1
- †The primary efficacy endpoint was the incidence of the adjudicated composite of recurrent symptomatic VTE or death from any cause related to VTE.1 Recurrent VTE included fatal or non-fatal PE and DVT.1
- ‡The primary safety endpoint was major bleeding.1 For patients who had more than one event, only the first was counted.1
- §The secondary safety endpoint was the composite of major or CRNM bleeding events.1 For patients who had more than one event, only the first was counted.1
- ¶This link is to a third-party site unaffiliated with Pfizer / BMS. Please abide by the terms of use of the site.
- BD = Twice Daily CI = Confidence Interval DVT = Deep Vein Thrombosis N = Total number of patients in either the ELIQUIS group or the placebo group n = Number of patients with event
- PE = Pulmonary Embolism RR = Relative Risk SmPC = Summary of Product Characteristics VTE = Venous Thromboembolism
References:
- Agnelli G et al. N Engl J Med 2013; 368: 699–708.
- ELIQUIS® (apixaban) Summary of Product Characteristics.
Reporting of suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance at www.hpra.ie
Adverse reactions should also be reported to Bristol Myers Squibb Medical Information on 1 800 749 749 or medical.information@bms.com
