


ELIQUIS (apixaban) dosing for patients with DVT / PE
ELIQUIS for the treatment of DVT / PE and prevention of recurrent DVT / PE
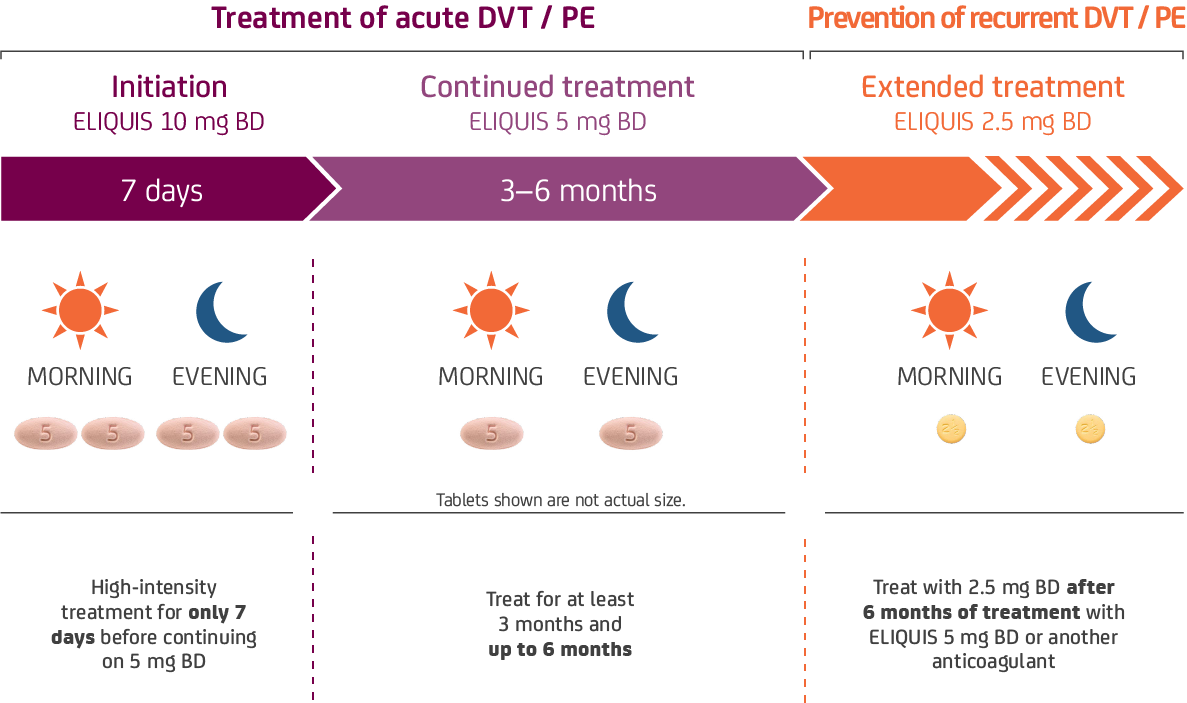
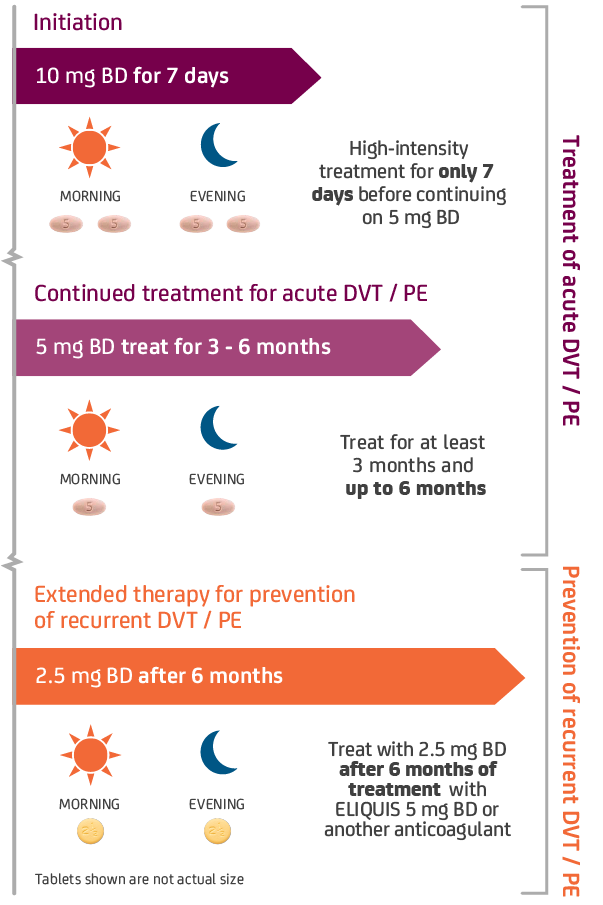
The duration of overall therapy should be individualized after careful assessment of the treatment benefit against the risk for bleeding, as per available medical guidelines, short duration of treatment (at least 3 months) should be based on transient risk factors (e.g. recent surgery, trauma, immobilisation).
Duration of overall treatment should be individualised after careful assessment of the treatment benefit against the risk for bleeding.1
No dose adjustment for DVT / PE patients, based on age, weight or those with mild-to-moderate renal impairment.1 ELIQUIS should be used with caution in patients with severe renal impairment (CrCl 15–29 ml/min) for the treatment of DVT / PE and prevention of recurrent DVT / PE.1 ELIQUIS is not recommended in patients with CrCl <15 ml/min, or in patients undergoing dialysis.1
ELIQUIS is a convenient, oral treatment for patients
No initial injections or bridging with LMWH required1
ELIQUIS can be taken
with or without food1
Missed dose: if a dose is missed,
the patient should take ELIQUIS
immediately and then continue
with twice-daily intake as before.1
Liver function testing should be performed prior to initiating ELIQUIS1
ELIQUIS (apixaban) is indicated for prevention of stroke and systemic embolism in adult patients with non-valvular atrial fibrillation (NVAF), with one or more risk factors, such as prior stroke or transient ischaemic attack (TIA), age ≥75 years, hypertension, diabetes mellitus, symptomatic heart failure (NYHA Class ≥II); treatment of deep vein thrombosis (DVT) and pulmonary embolism (PE), and prevention of recurrent DVT and PE in adults; and, prevention of VTE in adult patients who have undergone elective hip or knee replacement surgery.1
Footnotes:
- BD = Twice Daily CrCl = Creatinine Clearance DVT = Deep Vein LMWH = Low Molecular Weight Heparin PE = Pulmonary Embolism
References:
- ELIQUIS® (apixaban) Summary of Product Characteristics.
Reporting of suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via HPRA Pharmacovigilance at www.hpra.ie
Adverse reactions should also be reported to Bristol-Myers Squibb Medical Information on 1 800 749 749 or medical.information@bms.com
